Introduction
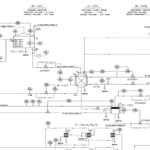
Marjan Petrochemical Methanol Unit is a part of Iran’s extensive petrochemical industry. And it plays a significant role in the production of methanol, a key chemical that is used as a feedstock in the production of other chemicals, fuels and materials. The methanol production process at these facilities, like most large-scale petrochemical plants, is generally classified into two main types: the traditional (industrial) process and more modern methods, often involving advanced technologies.
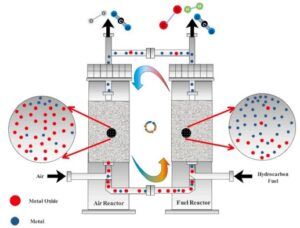
Mass production and emission of greenhouse gases have destroyed the planet Earth based on climate change and global warming. Carbon dioxide is known as one of the most important greenhouse gases. In recent decades, various methods have been developed for carbon capture and sequestration (CCS) along with the reduction of net CO2 production. Fossil fuels are the main sources of carbon dioxide emissions. Replacing fossil fuels with a clean and renewable energy source such as hydrogen is one of the promising ways to control CO2 emissions into the atmosphere.
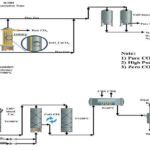
The increase in energy demand over the past few decades has reduced energy reserves. which has been largely offset by the consumption of fossil fuels that have increased greenhouse gas emissions. Therefore, the need for clean and sustainable alternatives to meet energy shortages has increased dramatically. Gasification and steam methane reforming (SMR) are effective technologies. which can produce synthetic gas and hydrogen from coal and natural gas respectively.
Comparison
– Industrial process: Traditionally, methanol is produced using natural gas (methane) as a feedstock. The process involves reforming natural gas with steam to produce syngas, a mixture of hydrogen, carbon monoxide and carbon dioxide.
– Modern process: Modern methods are also exploring the use of alternative raw materials,. such as renewable natural gas, biomass as part of the growing emphasis on sustainability. These raw materials can also be modified to produce synthesis gas. But the process may include additional steps to ensure the purity of the raw materials or the efficiency of the conversion.
Synthesis Gas Production
– Industrial process: Syngas production is traditionally done through steam methane reforming (SMR). This is a high temperature process. where natural gas reacts with steam over a catalyst to produce synthesis gas.
– Modern process: While SMR is still widely used. Newer technologies such as autothermal reforming (ATR) or partial oxidation (POX) are being used. These methods can be more energy efficient. And they may offer better integration with carbon capture technologies. For example, ATR combines both endothermic and exothermic reactions to reduce energy consumption.
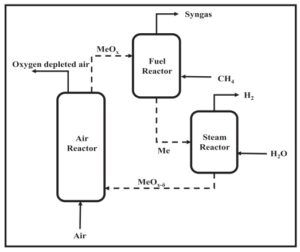
Methanol Synthesis
– Industrial process: In the traditional method, synthesis gas is passed over a copper-based catalyst at high pressure and temperature to produce methanol. The reaction is usually carried out in a fixed bed reactor.
– Modern process: Advanced catalysts and reactor designs are implemented in modern power plants to increase conversion rates and energy efficiency. Technologies such as slurry phase reactors or fluidized bed reactors are being explored for better heat management and improved reaction kinetics. In addition, the integration of pressure swing absorption (PSA) systems for syngas treatment increases the overall efficiency of the process.
Energy Consumption and Productivity
– Industrial process: Traditional methanol production is energy intensive, especially in the reforming and compression stages. Overall energy efficiency is often limited by the technology of the time.
– Modern process: Modern factories are designed with energy integration in mind. Innovations such as heat recovery systems, cogeneration and improved thermal management have significantly reduced energy consumption per unit of methanol produced. Some factories are also considering using renewable energy sources to further reduce their carbon footprint.
Environmental Considerations
– Industrial process: Historically, environmental concerns were secondary in methanol production. This process produces significant greenhouse gas emissions, especially from the refining stage.
– Modern process: There is a lot of focus on reducing the environmental impact. Modern facilities often use carbon capture and storage (CCS) technologies to reduce CO2 emissions.
Product Quality and Efficiency
Industrial process: In older plants, the yield and purity of methanol were often limited by the limitations of existing technologies. Achieving high purity requires additional purification steps.
– Modern Process: Technological advances have led to significant improvements in performance and purity. Modern catalysts and reactors are designed to maximize the conversion of syngas to methanol while minimizing byproducts. Advanced purification techniques ensure that the methanol produced meets stringent industry standards with fewer additional steps.
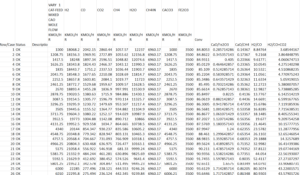
Sensitivity Analysis
The sensitivity analysis was done and all the graphs were designed in the Origin Lab Pro software.
Conclusion
The shift from traditional to modern methanol production processes at facilities such as the Marjan Petrochemical Methanol Unit is indicative of broader trends in the petrochemical industry: a focus on sustainability, efficiency and technological integration. While industrial processes laid the foundation for large-scale methanol production. Modern developments ensure that these processes are more environmentally friendly, cost-effective and compatible with future energy perspectives.
This project was carried out by SANILCO based on the following articles.
1. Simultaneous Production of Synthesis Gas and Hydrogen From Fossil and Biological Sources Using CO2
2. Thermodynamic Analysis of Simultaneous Production of H2 and Synthesis Gas From CO2
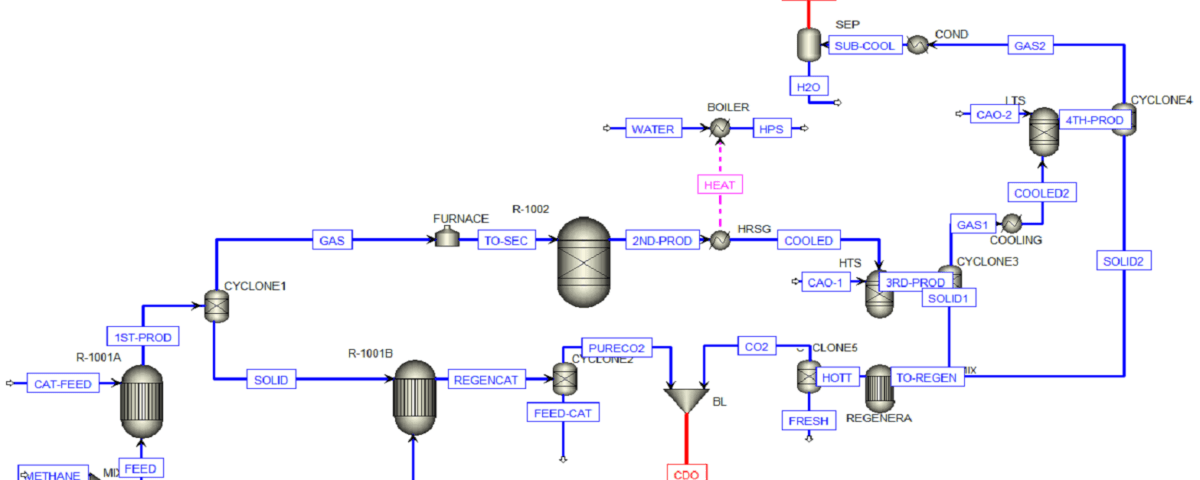
The conceptual design of a synthesis gas production process and its comparison with Marjan Petrochemical was carried out by SANILCO.
1. Full simulation of Marjan petrochemical methanol unit
2. Conceptual design of a new process to produce synthesis gas, hydrogen and absorb carbon dioxide
3. Comparison of synthesis and industrial gas production process of Marjan Petrochemical Methanol Unit
Innovative Syngas and Methanol Process vs Marjan Pet. Co. Method
In this project, Innovative Syngas and Methanol Process vs Marjan Pet. Co. Method has been done.