Butane Purification
مهر ۱۱, ۱۴۰۳
Sour Water Recovery Unit
مهر ۱۱, ۱۴۰۳Introduction
Caustic recovery is a chemical process in which sodium hydroxide (caustic soda) is produced through reduction, particularly in the chemical industry. This process is typically utilized for the purification and recovery of chemical materials, improving product quality, and reducing costs. In this process, raw materials containing sodium and water react under specific conditions, and through the use of electrical or thermal energy, sodium and hydroxyl ions are liberated.
The importance of caustic recovery is particularly notable in the industries of pulp and paper, paints, and other chemical products, as caustic soda is a key raw material in many processes. Additionally, caustic recovery is regarded as a sustainable method for waste management and resource recovery, given its potential to reduce the consumption of raw materials and preserve the environment. With the increasing demand for caustic soda and the environmental issues related to its production, research into optimizing and improving the caustic recovery process is expanding to maximize the efficiency and profitability of industrial processes.
Caustic Recovery Unit
The main goal of the caustic recovery unit is to economically and sustainably produce and recover sodium hydroxide (caustic soda). The primary objectives of this unit include:
1. Continuous and Quality Production: Establishing a process capable of producing high-quality sodium hydroxide while ensuring sustainable production.
2. Reducing Raw Material Consumption: Optimizing the recovery process to decrease the use of raw materials and energy, aiming to enhance efficiency and reduce costs.
3. Environmental Protection: Minimizing the environmental impacts associated with caustic production through process optimization and the use of clean technologies.
4. Increasing Productivity and Competitiveness: Empowering the production unit to boost its market competitiveness, particularly in response to the changing needs of industries.
Overall, the caustic recovery unit strives to provide a sustainable and optimized production of caustic soda with economic and environmental goals in mind.
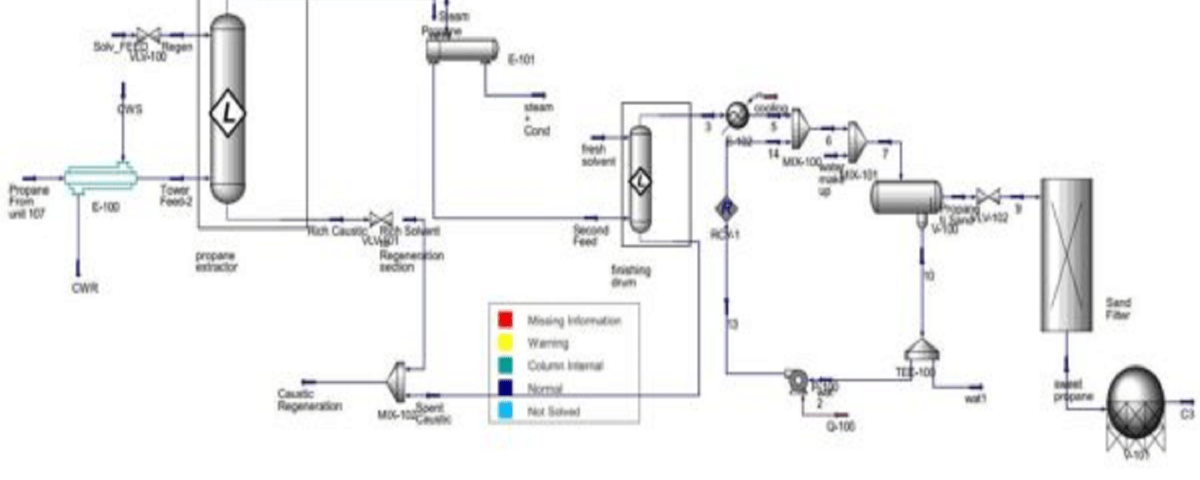
The main goal of the caustic recovery and purification unit is to isolate mercaptans from sour propane and butane gases produced by unit 107. The input materials for this unit comprise caustic solution and disulfides obtained from units 114 and 115. This process converts the mercaptans dissolved in caustic into DSO or disulfide and water.
Absorption Reactions:
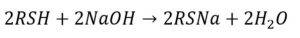
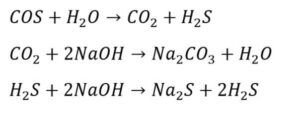
– Reduction Reactions:
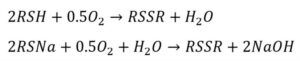
This reaction takes place with the injection of air in the oxidizer, using a catalyst known as LCPS30. After the reaction, a separator allows for the separation of disulfide and light caustic. The disulfide, being less dense, rises and is sent to unit 146 for storage, where it is used in certain chemical workshops. Additionally, excess air that separates from light caustic, referred to as Spend Air, is collected from the top of the disulfide separator and directed towards unit 121. Each phase includes two similar units.
Conclusion
This process is of great significance as a sustainable and efficient method for producing sodium hydroxide. With the rising demand for caustic soda in various industries such as pulp and paper, chemicals, and food processing, caustic recovery can aid in reducing reliance on new raw material resources, and subsequently, lessen costs and the environmental impacts caused by its extraction and production. Optimizing the recovery process with a focus on the use of modern technologies to improve efficiency and reduce energy and raw material consumption is vital not only from an economic perspective but also in terms of environmental protection.