Introduction
This project investigates corrosion in a steel pipe containing a flowing aqueous solution of carbon dioxide. In this one-dimensional model, it is assumed that the physical properties of the pipe remain constant along its length. Additionally, the boundary layer thickness is dependent on the Reynolds number. All species present are considered soluble in water, and water is the dominant component in the mixture. This assumption leads to mass transfer occurring solely through diffusion, with convective mass transfer neglected in this model. Diffusion coefficients for the species involved in the reactions and mass transfer are provided in Table 1.
Equilibrium Reactions
Several equilibrium reactions occur within the electrolyte. These reactions include:
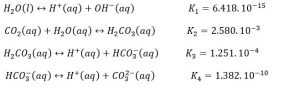
These equilibrium reactions are the result of the solubility of carbon dioxide in water, whose reaction is as follows:
![]()
Although dissolved CO2 is not a corrosive species by itself, it causes the solution to become acidic. The dissolution of carbon dioxide gas in water causes the formation of a series of homogeneous reactions that occur simultaneously and in series.
Fe(s) -> Fe2+ + 2e-
H+ + e–> H
H2O + e–> H + OH-
H2CO3 + e–> H + HCO3-
HCO3- (aq) + e–> 1/2 CO2 + 1/2 H2(g)
Modeling of Corrosion Reactions with COMSOL
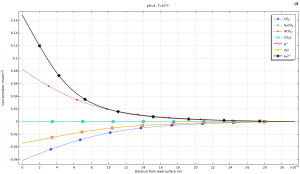
In this project, the modeling of corrosion reactions has been done in Comsol software. The figure below shows the concentration of components at a short distance from the wall.This project has a full report and an educational video.