The Importance of Fuel Oil Desulfurization
Fuel oil is one of the primary products of crude oil refining, widely used as fuel in maritime transportation, power plants, and various industrial processes. However, it contains significant amounts of sulfur compounds, which, during combustion, are converted into sulfur dioxide (SO₂). This leads to severe environmental impacts, including air pollution, acid rain, and increased corrosion of industrial equipment. Consequently, global regulatory bodies such as the International Maritime Organization (IMO 2020) and stringent EURO V standards have mandated the desulfurization of fuel oil. In response, refineries have adopted advanced technologies to remove sulfur compounds efficiently while ensuring both environmental compliance and economic feasibility.
Objectives of the Fuel Oil Desulfurization Project at Lavan Refinery
The project titled “Process Design, Simulation, and Technical Proposal for Fuel Oil Desulfurization at Lavan Refinery” aims to develop an innovative and efficient solution for reducing sulfur compounds in fuel oil and optimizing refining processes. The key objectives include:

- Reducing sulfur content in fuel oil to meet international regulatory limits.
- Enhancing product quality by optimizing refining processes and improving technical and performance indices.
- Minimizing environmental pollutants and mitigating the negative environmental effects of fuel oil combustion.
- Increasing refinery efficiency through modern technology, reducing energy consumption, and optimizing production processes.
Technical and Innovative Approach in Process Design
This project employs state-of-the-art process engineering methodologies and advanced simulation software. Unlike conventional methods, a hybrid and optimized approach is implemented, integrating two key technologies:
- Hydrodesulfurization (HDS): In this process, sulfur compounds are converted into hydrogen sulfide (H₂S) in the presence of hydrogen and a suitable catalyst, effectively removing sulfur from the feedstock.
- Oxidative Desulfurization (ODS): Residual sulfur compounds are oxidized using hydrogen peroxide (H₂O₂) and subsequently extracted via liquid-liquid extraction (LLE).
This integrated approach ensures higher efficiency in sulfur removal, resulting in a cleaner, environmentally friendly fuel.
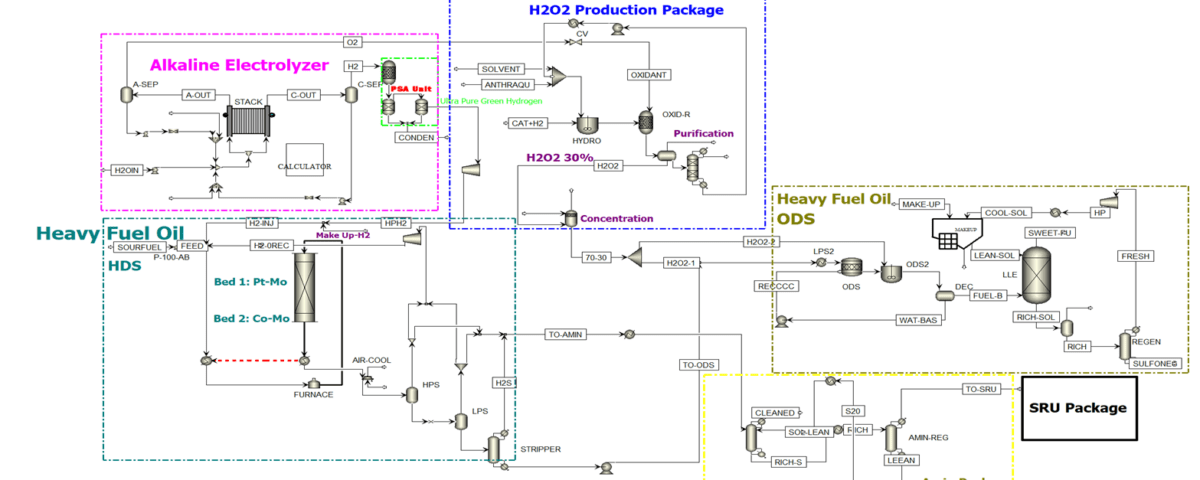
Key Process Units in Fuel Oil Desulfurization
The design of this unit comprises four critical sections, each playing a fundamental role in sulfur compound removal and process optimization:
-
Alkaline Electrolyzer:
- Produces high-purity hydrogen via alkaline electrolysis.
- Supplies hydrogen gas for downstream units (HDS and ODS).
-
Hydrogen Peroxide Production Unit:
- Generates 30% H₂O₂ for oxidative desulfurization.
- Utilizes the anthraquinone process for stable and safe H₂O₂ production.
-
Hydrodesulfurization (HDS) Unit:
- Reduces sulfur content by reacting with hydrogen in the presence of a catalyst.
- Separates H₂S and transfers it to sulfur recovery units.
-
Oxidative Desulfurization (ODS) Unit:
- Completes sulfur removal using H₂O₂.
- Employs liquid-liquid extraction (LLE) for oxidation product removal.
These four units operate in synergy to produce a final product—low-sulfur fuel that complies with environmental standards.
Advanced Fuel Oil Desulfurization at Lavan Refinery
With the increasing stringency of environmental regulations such as IMO 2020 and EURO V, refineries must adopt advanced technologies to reduce sulfur content in fuel oil. This project presents an innovative and hybrid approach for fuel oil desulfurization at Lavan Refinery, optimizing energy consumption, reducing operational costs, and minimizing environmental impact.
This hybrid process significantly lowers sulfur compound levels, producing an environmentally friendly fuel that meets strict international standards. Below, each of these process units is examined in further detail.
Alkaline Electrolyzer Unit
The alkaline electrolyzer unit is responsible for producing high-purity hydrogen required for desulfurization processes. This technology uses electric current in an alkaline medium (KOH or NaOH) to split water into hydrogen and oxygen.
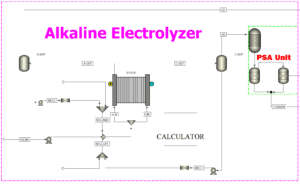
Inputs:
- Purified water (H₂O): Treated through a water purification system before electrolysis.
- Electrical energy: Provides the driving force for water decomposition.
Outputs:
- High-purity hydrogen (H₂): Sent to the PSA unit to achieve a purity level of over 99.99%.
- Oxygen (O₂): Used for industrial applications or safely vented.
Process Description:
- Purified water passes through ion-exchange and filtration systems.
- The treated water enters the alkaline electrolyzer, where direct current (DC) is applied, splitting water into hydrogen and oxygen.
- Hydrogen ions (H⁺) receive electrons at the cathode, forming H₂ molecules.
- Hydroxide ions (OH⁻) lose electrons at the anode, producing O₂.
- The produced hydrogen is separated using gas-liquid separators and sent to the PSA unit for further purification before use in the HDS process.
Advantages of This Technology:
- Produces green hydrogen without reliance on fossil fuels.
- Reduces operational costs compared to traditional hydrogen production methods.
- Environmentally friendly with zero greenhouse gas emissions.
Hydrogen Peroxide Production Unit
The hydrogen peroxide (H₂O₂) production unit plays a crucial role in this process. H₂O₂, a strong oxidizing agent, converts residual sulfur compounds into sulfones and sulfur oxides, facilitating their removal.
Inputs:
- High-purity hydrogen (H₂): Supplied from the PSA unit.
- Anthraquinone solvent: Used as a reaction medium for H₂O₂ synthesis.
- Oxidant: Activates the oxidation reaction.
Outputs:
- 30% hydrogen peroxide (H₂O₂) solution: Used in the oxidative desulfurization (ODS) unit.
- Byproduct gases: Sent to the recovery system.
H₂O₂ Production Process:
- Hydrogen from the PSA unit is introduced into a catalytic oxidation reactor, reacting with anthraquinone under controlled pressure.
- Hydrogenated anthraquinone, in the presence of an oxidant, produces H₂O₂.
- The generated H₂O₂ is purified and filtered before being transferred to storage tanks.
Advantages of This Technology:
- Enables stable and safe in-house production of H₂O₂, reducing dependency on imports.
- Lowers operational costs and enhances refinery self-sufficiency.
- Optimizes ODS efficiency by ensuring a steady oxidant supply.
These two units—alkaline electrolysis and H₂O₂ production—play a vital role in reducing sulfur compounds in fuel oil. By generating pure hydrogen and producing H₂O₂ in-house, Lavan Refinery can execute the desulfurization process with high efficiency, lower operational costs, and minimal environmental impact.
Hydrodesulfurization Unit (HDS – Hydrodesulfurization)
The Hydrodesulfurization Unit (HDS) is one of the key stages in the process of sulfur reduction from fuel oil at the Lavan Refinery. This unit utilizes catalytic hydrogenation to convert sulfur compounds present in fuel oil into hydrogen sulfide (H₂S), thereby reducing the sulfur content in the final fuel product.
The following diagram summarizes the kinetic coefficients of HDS reactions for benzothiophene and the rate consumption formulas for DBT:
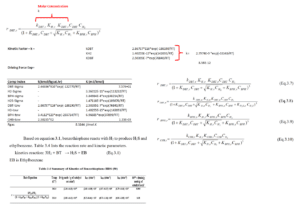
Main Objectives of the HDS Unit:
- Reduction of sulfur content in fuel oil
- Enhancing fuel quality and ensuring compliance with international environmental standards
- Improving combustion efficiency and reducing sulfur-based pollutants (SO₂ and SO₃)
Process Inputs:
- Heavy fuel oil feed (SOUR FUEL): Containing heavy sulfur compounds such as mercaptans, thiophenes, and benzothiophenes
- Hydrogen gas (H₂-INJ): Supplied from the alkaline electrolyzer and PSA unit
- Heat provided by the furnace: To activate hydrogenation reactions
Process Outputs:
- Fuel oil with reduced sulfur content: With a lower sulfur percentage, further processed in the Oxidative Desulfurization (ODS) unit
- H₂S gas: Sent to the stripper unit for separation from the fuel stream and utilized for sulfur recovery in the Claus unit
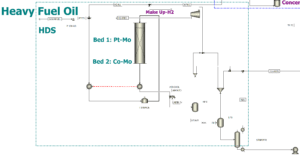
HDS Process Steps:
- Feed Preheating: The fuel oil is heated to 350–400°C through heat exchangers and a furnace before entering the reactor.
- Two-Bed Reactor Reaction:
- First Bed (Pt-Mo Catalyst): Removes heavy sulfur compounds like dibenzothiophenes (DBT)
- Second Bed (Co-Mo Catalyst): Completes desulfurization reactions, converting remaining sulfur compounds into H₂S
- H₂S Gas Separation: The output stream is cooled and passed through gas-liquid separators, sending H₂S to the stripper unit for sulfur recovery.
- Product Transfer to the ODS Unit: The desulfurized fuel oil enters the Oxidative Desulfurization (ODS) unit for further sulfur removal.
Advantages of HDS Technology:
- High efficiency in sulfur compound removal
- Compatibility with high-sulfur crude oil feedstocks
- Integration with ODS processes to achieve sulfur levels below 0.5% by weight
Oxidative Desulfurization Unit (ODS)
The Oxidative Desulfurization (ODS) unit is designed as the final stage of the desulfurization process to remove refractory sulfur compounds from fuel oil. Unlike the Hydrodesulfurization (HDS) process, which relies on hydrogen and high pressure, ODS uses controlled oxidation to convert the remaining sulfur compounds into sulfones, which are then separated from the fuel stream.
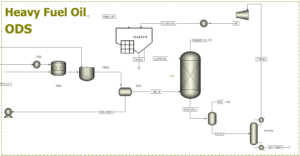
Importance of ODS:
- Removes hard-to-eliminate sulfur compounds such as dibenzothiophenes that HDS cannot fully eliminate.
- Operates under milder conditions compared to HDS (lower pressure and temperature).
- Optimizes the desulfurization process without excessive hydrogen consumption.
Process Inputs:
- Reduced sulfur fuel oil: Output from the Hydrodesulfurization (HDS) unit.
- H₂O₂ solution (30%): Supplied by the Hydrogen Peroxide Production Package.
- Special separation solvents: Facilitate the extraction of sulfone compounds.
Process Outputs:
- Cleaned Fuel: With sulfur content lower than 0.5% by weight.
- Sulfone solution: Sent for recovery or proper disposal.
ODS Process Steps:
- Oxidation of Sulfur Compounds: Fuel oil from the HDS unit is exposed to H₂O₂ and a special acidic catalyst. Refractory sulfur compounds like dibenzothiophenes (DBT) and benzothiophenes (BT) are oxidized to sulfones.
- Liquid-Liquid Extraction (LLE): The now more polar sulfone compounds dissolve in special separation solvents and are extracted from the fuel oil.
- Solvent Recovery and Final Purification: After sulfones are removed, solvents are recycled back into the process, and the clean fuel is sent for storage and use.
Advantages of ODS Technology:
- Enables deep desulfurization without high pressure.
- Eliminates dependence on hydrogen, reducing energy consumption.
- Improves fuel quality and reduces environmental pollutants.
By integrating HDS and ODS, Lavan Refinery can produce low-sulfur fuel oil with maximum efficiency while reducing operational costs and fully complying with global standards.
Amine Package Unit
The Amine Package Unit is one of the key units in the oil refining process, responsible for removing hydrogen sulfide (H₂S) from gas streams. This step is crucial for preventing the release of harmful sulfur gases and providing a suitable feed for the Sulfur Recovery Unit (SRU).
Primary Objectives of the Amine Package:
- Remove H₂S and CO₂ from refinery gas streams.
- Provide a purified feed for the Sulfur Recovery Unit (SRU).
- Reduce sulfur-based pollutants and improve refinery environmental performance.
Process Inputs:
- H₂S-containing gas: Output from the Hydrodesulfurization (HDS) unit.
- Absorbing amine solution: Includes Monoethanolamine (MEA), Diethanolamine (DEA), or Methyl Diethanolamine (MDEA).
Process Outputs:
- Purified gas: Suitable for discharge or use in other processes.
- H₂S-rich solution: Sent to the SRU for sulfur recovery.
Amine Package Process Steps:
- H₂S Gas Absorption: The gas stream from the HDS unit enters the absorber column, where it contacts the amine solution. H₂S and CO₂ are absorbed, and the treated gas exits from the top of the column.
- Amine Solution Regeneration: The H₂S-rich amine solution moves to the regenerator column, where heat and reduced pressure separate H₂S from the amine. The regenerated amine is reused in the absorption process.
- H₂S Transfer to SRU: The separated H₂S gas is sent to the Sulfur Recovery Unit (SRU) for conversion into elemental sulfur.
Advantages of the Amine Package Technology:
- High efficiency in removing H₂S from refinery gases.
- Reduction of sulfur gas emissions and environmental pollution.
- Ability to recover and reuse the amine solution.
Sulfur Recovery Unit (SRU)
The Sulfur Recovery Unit (SRU) plays a vital role in managing sulfur produced in refinery processes. This unit converts H₂S gas from the Amine Package into high-purity elemental sulfur, which is used in various industries, including sulfuric acid production, agriculture, and chemicals manufacturing.
Primary Objectives of the SRU:
- Recover sulfur from acid gases and reduce sulfur pollution.
- Comply with environmental regulations by minimizing SO₂ emissions.
- Produce high-purity sulfur for industrial applications.
Process Inputs:
- H₂S-rich gas: Output from the Amine Package.
Process Outputs:
- Pure elemental sulfur: Stored in molten form and sent for industrial use.
- Off-gases: Treated further to minimize emissions.
SRU Process Steps (Claus Process):
- Thermal Stage (Combustion):
- H₂S gas is burned in a reactor furnace with a controlled oxygen supply.
- This reaction converts part of the H₂S to SO₂ and some to molten sulfur.
- Catalytic Stage:
- The gases pass through catalyst beds where the Claus reaction continues, converting remaining SO₂ into elemental sulfur.
- Sulfur Separation and Storage:
- Produced sulfur is separated using condensers and transferred to storage units.
- Final Off-Gas Treatment:
- Remaining gases (containing trace H₂S and SO₂) are processed in a Tail Gas Treatment Unit to minimize environmental impact.
Advantages of SRU Technology:
- Maximized sulfur recovery from acid gases.
- Reduced SO₂ emissions for improved environmental compliance.
- Production of high-purity sulfur for industrial applications.
With the integration of the Amine Package and SRU, Lavan Refinery effectively reduces sulfur emissions while recovering valuable sulfur for industrial use.
Conclusion
This innovative process, combining modern desulfurization methods (HDS + ODS), alkaline electrolysis for hydrogen and H₂O₂ production, and optimized sulfur recovery (Amine + SRU), provides an efficient, cost-effective, and sustainable solution for Lavan Refinery. It not only reduces environmental pollutants but also enhances the quality of fuel oil.
The project has delivered remarkable results in reducing pollutants, improving product quality, and optimizing industrial performance. Key achievements include:
- Reduction of Sulfur Content in Fuel Oil:
- Sulfur content reduced from 3.5% to below 0.5%, meeting IMO 2020 fuel regulations.
- Improved engine performance and reduced emissions of SO₂ and SO₃.
- Minimized corrosion and sulfur deposits in equipment and pipelines.
- Enhanced Industrial Process Efficiency:
- Optimized hydrogen consumption and reduced operational costs.
- Shorter processing times and increased reaction efficiency.
- Capability to refine various fuel oils with different sulfur contents.
- Reduction of Environmental Pollutants:
- Lower SO₂ and SO₃ emissions, reducing acid rain formation.
- Alignment with the Paris Agreement and greenhouse gas reduction policies.
- Decreased sulfur-contaminated industrial wastewater.
- Economic Efficiency and Cost Reduction:
- Lower hydrogen costs through in-house production.
- Increased value of desulfurized fuel in global markets.
- New export opportunities for low-sulfur fuel and sulfur by-products.
- Energy Optimization and Process Sustainability:
- Reduced electricity consumption via optimized heat exchange systems.
- Lower natural gas consumption in steam production.
- Improved process stability using automation and AI-based controls.
- Recovery and Reuse of By-Products:
- Elemental sulfur used in chemical, agricultural, and sulfuric acid industries.
- Recycled H₂S gas used as feedstock for the SRU.
- Oxygen from electrolysis used in combustion and industrial purification processes.
By successfully implementing these advanced technologies, Lavan Refinery sets a benchmark for producing cleaner and more sustainable fuels while reducing environmental impact.
Compliance with International Standards
This project has successfully obtained certifications for low-sulfur fuel production by adhering to recognized international standards.
Standards Implemented:
- IMO 2020 – International Maritime Organization standards for reducing sulfur content in marine fuels
- EURO V & EURO VI – Standards for limiting sulfur-based emissions in transportation fuels
- ISO 8217 – International quality standard for marine fuels
Related Project
Design and Simulation of Fuel Oil Desulfurization Process at Lavan Refinery
Project Summary
The Lavan Refinery Fuel Oil Desulfurization Project, leveraging innovative technologies and advanced process designs, represents a significant step towards producing low-sulfur and environmentally friendly fuels. This project has not only substantially reduced sulfur content in fuel oil but has also optimized energy consumption, enhanced economic efficiency, and minimized environmental pollutants.
By combining Hydrodesulfurization (HDS) and Oxidative Desulfurization (ODS) processes, the sulfur content in fuel has been reduced below IMO 2020 standards, improving the final product’s quality. Additionally, in-house production of hydrogen and hydrogen peroxide has lowered operational costs while improving process stability and efficiency.
Furthermore, the reduction of SO₂ emissions, enhanced refinery equipment performance, and extended equipment lifespan highlight this project’s positive impact on environmental protection and sustainable development. The recovery of by-products such as elemental sulfur and industrial oxygen has minimized waste while creating new economic opportunities for the refinery.
Ultimately, this project serves as a successful model for other refineries, paving the way for the production of cleaner and more sustainable fuels. The adoption of smart technologies and continuous process optimization will further facilitate the global transition to ultra-low-sulfur fuels and mitigate environmental impacts.