Introduction
Ethyl acetate is one of the most important industrial esters, widely used in the chemical, pharmaceutical, and food industries, as well as a solvent in the production of paints, adhesives, and lacquers. This compound is typically produced through the esterification reaction between acetic acid and ethanol in the presence of an acid catalyst, and the method of reactive distillation has gained attention.
Reactive distillation is an advanced technology that combines chemical reaction and separation in a single operational unit. This method not only increases process yields by shifting chemical equilibrium but also reduces energy consumption, capital and operational costs, and waste generation. In this project, Aspen Plus software has been utilized for the simulation and optimization of ethyl acetate production via reactive distillation.
Ethyl Acetate
Ethyl acetate is one of the simplest carboxylate esters and is an environmentally friendly organic solvent used in paints and adhesives, thereby eliminating the use of aromatic compounds in the workplace. This is widely employed as a solvent in chemical reactions or preparations, which is why it is produced on a large scale. It is also known as ethyl ethanoate and is commonly abbreviated as EtOAc. This organic compound is primarily used as a solvent in various reactions. The developed formula for ethyl acetate is CH₃COOCH₂CH₃. Ethyl acetate is highly flammable and is generally utilized as an organic solvent in paints, films, cleaning products, and more.
Introduction to Reactive Distillation Process
Reactive distillation is a process in which chemical reactions and the separation of products are carried out in a single operational unit. This method is particularly suitable for reactions that are limited by chemical equilibrium. such as the production of ethyl acetate. In the production of ethyl acetate, the esterification reaction occurs between acetic acid and ethanol in the presence of an acid catalyst (such as sulfuric acid).
CH3COOH+C2H5OH↔CH3COOC2H5+H2OCH3COOH+C2H5OH↔CH3COOC2H5+H2O
In the reactive distillation process, the reaction products (ethyl acetate and water). are simultaneously separated from the reaction environment, which shifts the equilibrium of the reaction toward increased product formation.
Advantages of Reactive Distillation
Increased Process Yield: By removing products from the reaction environment, the chemical equilibrium shifts toward greater product formation.
Reduced Energy Consumption:
Conducting the reaction and separation in a single operational unit reduces energy costs.
Lower Capital and Operating Costs:
This approach requires less equipment and simplifies the process.
Minimized Waste:
Due to the high yield, waste production is kept to a minimum.
Modification of Conventional Ethyl Acetate Production Using Reactive Distillation Column
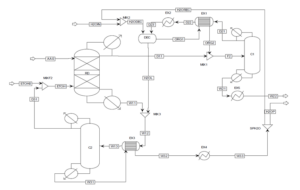
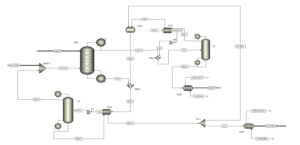
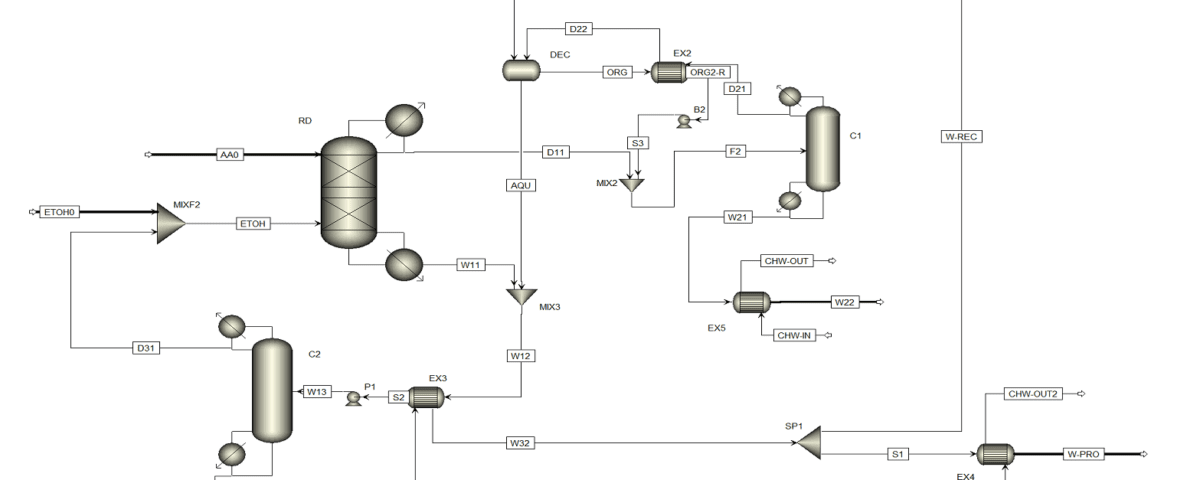
The improvement in the ethyl acetate production process is achieved by integrating reaction and separation through the reactive distillation process. Several reactive distillation (RD) plants for ethyl acetate production operate globally, with an annual production capacity of 20,000 tons. As RD is a multi-functional reactor concept that combines the mechanisms of reaction and separation in a single unit, it results in benefits such as a reduction in the number of equipment, unit size, and improved process efficiency, leading to enhanced process economics. The CSTR columns, EX1, and C1 are placed together in a single RD column, resulting in the elimination of the reactor (CSTR) and two heat exchangers compared to the previous process.
Feed Stream of The Reactive Distillation Column
The reactive distillation column has two feed streams: acetic acid (AA0) and ethanol (ETOH). In this process, all the acetic acid is consumed in the RD. Therefore, the overhead stream of the RD column (D11) contains an almost azeotropic mixture of water, ethyl acetate, and ethanol, while the bottom product (W11) contains a mixture of water and ethanol. The composition of the overhead stream from the reactive distillation column is such that it can be directly separated. C1 column is employed to isolate pure ethyl acetate. Bottom product of column C1 (W21) contains pure ethyl acetate, which is cooled in the heat exchanger EX5. A ternary azeotrope of water, ethyl acetate, and ethanol exists in the distillation column C1.
Therefore, columns C1 and C2 in the first process state operate similarly. The distillate stream D21 is cooled in heat exchangers EX1 and EX2 and then fed into the decanter for ethyl acetate extraction. Pure water is fed into the DEC, where the organic and aqueous phases are separated. The organic phase is preheated in EX1, while the aqueous phase contains some ethanol and ethyl acetate. To recover water, distillation column C2 is used. All ethanol and ethyl acetate are obtained in the overhead stream of column C2 (D31), which is recycled back to the RD column. Pure water is separated in the bottom product of C2 (W31) and cooled in EX3 and EX4. A molar ratio of water to ethyl acetate product (W22) is separated as the final product (H2OP), and the remaining water is recycled back to the decanter (H2OREC).
Simulation in Aspen Plus
Aspen Plus is one of the most powerful tools for simulating chemical processes, used for modeling and optimizing complex processes such as reactive distillation. The steps involved in simulating the production of ethyl acetate via reactive distillation in Aspen Plus are as follows:
Definition of Chemical Compounds
Initially, the chemical compounds used in the process (acetic acid, ethanol, ethyl acetate, and water) are introduced into the software. These compounds are selected from the internal libraries of Aspen Plus.
Selection of Thermodynamic Model
Choosing an appropriate thermodynamic model for simulation is crucial. For systems containing esters and alcohols, models such as NRTL (Non-Random Two-Liquid) or UNIQUAC are commonly used. These models are suitable for predicting phase behavior and liquid-vapor equilibrium.
Design of The Reactor-Distillation Column
In Aspen Plus, the reactor-distillation column is defined as a single operational unit. This unit includes the following sections:
Reaction Zone: Where the esterification reaction takes place.
Separation Zone: Where the products (ethyl acetate and water) are separated from the remaining reactants.
Simulation of The Reactive Distillation Column
Determination of Number of Stages: The number of distillation stages and the location for the injection of feed materials must be established.
Determination of Reaction Location:
The chemical reaction typically occurs in the middle stages of the column.
Calculation of Phase and Reaction Equilibrium:
Using appropriate thermodynamic models (such as NRTL or UNIQUAC), the phase and reaction equilibrium must be calculated.
Determination of Operating Conditions:
Operational parameters such as temperature, pressure, molar ratio of reactants, and reflux ratio are entered into the software. These parameters have a direct impact on process yield and the purity of the final product.
Execution of Simulation and Analysis of Results:
After all parameters are established, the simulation is executed. The results include the yield of ethyl acetate production, product purity, energy consumption, and other key parameters. These results are utilized for process optimization.
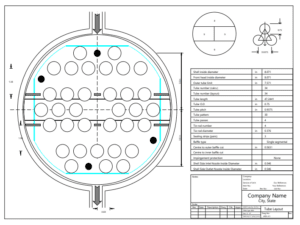
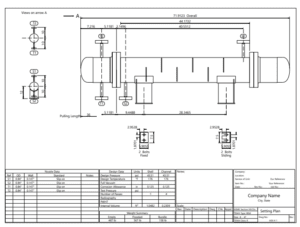
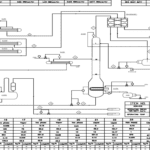
Design of Heat Exchangers
The design and fabrication drawings of the heat exchangers and columns used in the production of ethyl acetate via reactive distillation require thorough analysis and consideration of process requirements. This method can lead to improved production yields and reduced costs due to the simultaneous occurrence of chemical reactions and distillation.
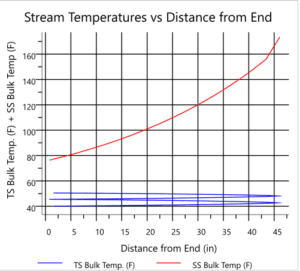
Heat exchangers are designed as one of the key components in the process. These exchangers must effectively transfer heat to maintain the appropriate temperature and pressure for both the reaction and distillation. Selection of the type of exchanger, dimensions, and materials of construction are critical aspects of the design. Design must include precise calculations for heat transfer and pressure drop to achieve optimal performance.
The images below pertain to heat exchangers used in the ethyl acetate production unit.
Anil Pars Process Industry Company
Anil Pars Process Industry Company is recognized as one of the leading firms in the design and simulation of chemical processes, having undertaken numerous projects in the field of ethyl acetate production, including via reactive distillation. Leveraging the expertise of seasoned engineers and utilizing advanced software, the company meticulously designs heat exchangers, reactive distillation columns, and simulates ethyl acetate production units with high precision and quality. Anil Pars Company provides accurate and optimized engineering solutions, assisting chemical industries in executing their processes with higher yields and lower costs. The company also conducts economic and technical analyses to ensure that the designs offered are technically feasible and economically viable.
Sample Projects
Simulation of 2400 Tons/Year Ethyl Acetate Production Unit
Design, Simulation and FS of 100KTY Ethyl Acetate Complex
Conclusion
The simulation of ethyl acetate production via reactive distillation demonstrates that this method is an efficient and cost-effective process for producing ethyl acetate. Reactive distillation, by combining chemical reaction and separation in a single operational unit, offers significant advantages, including reduced energy consumption, lower capital and operating costs, and increased process yield. The simulation of this process has been carried out using Aspen Plus software, and by employing EDR in the design of heat exchangers, optimal design of reactive distillation columns and heat exchangers can be achieved.