Introduction
The simulation and economic evaluation of carbon dioxide capture after combustion using piperazine in
Aspen Plus systems is an important topic in the field of pollution control and climate change. Given the increase in greenhouse gas emissions and their negative effects on the environment, the development of effective technologies for carbon dioxide capture and reduction has become a necessity.
Piperazine has gained attention as an efficient absorbent in carbon dioxide capture processes. Due to its specific chemical and physical properties, this compound has a high capability for absorbing CO2 from combustion flue gases. Simulating this process with the help of chemical engineering software allows for a precise analysis of system behavior and the evaluation of the performance of various absorbents. These simulations can aid in optimizing operational conditions, determining economic costs, and assessing the effectiveness of different capture methods.
Process Description
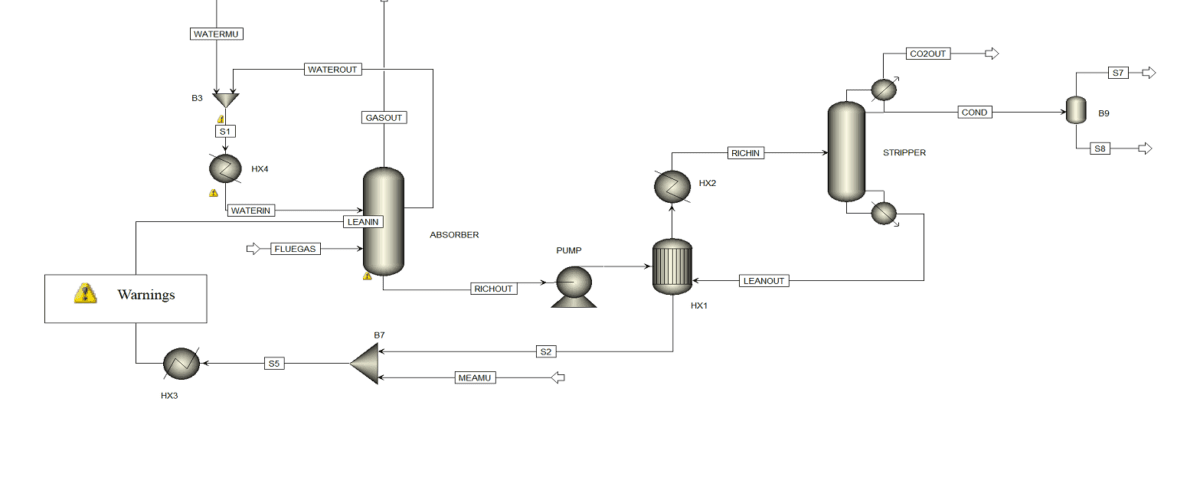
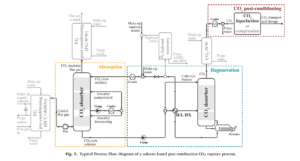
The process of carbon dioxide (CO2) capture has been investigated using an advanced configuration that differs from conventional solvent-based processes. This configuration includes two key features: an internal cooling absorbent and a pumparound stream, along with rich solvent splitting (RSS) in the absorption and regeneration sections.
This process, following combustion, typically consists of several main sections that can be applied to most solvent systems. These sections generally include the following:
Absorption Section
In this section, a portion of the CO2 present in the flue gases (FG) is transferred to a liquid solvent solution. This process helps to reduce the CO2 concentration in the flue gases.
Regeneration Section
In this stage, the absorbed CO2 is separated from the solvent using thermal energy, typically provided by steam. This action regenerates the diluted solvent that was used in the absorption section.
CO2 Pre-Conditioning Section
In this section, the CO2 produced in the regeneration stage is compressed and, if necessary, liquefied to prepare it for transportation. Depending on the characteristics of the incoming flue gas and the volatility and degradation properties of the solvent under normal operating conditions, additional sections may be required to remove impurities, condition the gases, and recover and recycle the solvents. These additional sections typically include the following:
FG Pre-Conditioning Section
In this section, the flue gases produced from the CO2 source are cooled and, if necessary, purified from impurities such as SOx and NOx.
CO2 Water Washing Section
This section is used for further cleaning of the CO2 gas stream before entering the CO2 pre-conditioning section, especially in cases where the solvent has high volatility.
Solvent Recovery Section
This section is designed to remove heavy compounds resulting from the degradation of amines and/or stable heavy salts obtained from the absorption of impurities. The recovered solvent is returned to the absorption-regeneration cycle.
The use of internal cooling in the CO2 absorber also increases the driving force for the transfer of CO2 from gas to liquid and extends the solvent’s residence time. These changes lead to a reduction in the steam requirement for solvent regeneration and a decrease in the flow rate of the aqueous solution between the absorber and the regenerator.
This advanced configuration consumes less energy compared to conventional methods and can reduce the size of the absorber packages. as well as enhance CO2 solubility and improve process efficiency. Additionally, rich solvent splitting (RSS) aids in better temperature control in the CO2 regenerator and minimizes the thermal load.
Process Modeling
In this article, the process of modeling post-combustion carbon dioxide absorption using an aqueous piperazine solution is examined in detail. The key points discussed are as follows:
1. The process modeling was conducted using Aspen Plus software version 8.6. In this modeling, the RateSep model was employed to simulate the carbon dioxide absorption column.
2. The NRTL electrolyte thermodynamic model and the PC-SAFT equation of state were used to calculate activity coefficients and vapor phase fugacity coefficients.
3. The equilibrium constants for the chemical reactions related to the reaction of carbon dioxide with piperazine were extracted from previous studies.
4. The mass and energy transfer model was implemented using correlations available in the scientific literature.
5. The column absorption modeling was validated using experimental data from existing pilot studies in the literature. The results indicate that the model is capable of accurately predicting carbon dioxide absorption efficiency and temperature profiles.
6. The operation of other equipment such as pumps, flue gas inlet fans, carbon dioxide compressors, coolers, and rich-lean heat exchangers was also simulated using existing models in Aspen Plus.
7. The modeling of the stripping column was considered under equilibrium assumptions, given the temperatures above 150 degrees Celsius, which accelerate the reaction and mass transfer rates.
Simulation
The simulation of carbon dioxide (CO2) absorption post-combustion using piperazine in Aspen Plus is conducted to investigate the efficiency and effectiveness of this substance in absorbing greenhouse gases. Piperazine is used as a chemical absorbent in this process, reacting with CO2 to form stable compounds.
System Definition
Modeling the combustion conditions and CO2 emissions, along with the characteristics of piperazine and its interaction with CO2.
Thermodynamic Modeling
Utilization of thermodynamic equations to predict the behavior of piperazine in CO2 absorption and to determine optimal conditions.
Fluid Dynamics Simulation
Examination of gas flow and the distribution of CO2 within the system through dynamic simulations.
Output Analysis
Evaluation of the absorption efficiency of piperazine and comparison with other absorbents, as well as an investigation of factors such as temperature and pressure on the absorption process.
By conducting this simulation, a better understanding of the CO2 absorption process can be achieved, leading to the optimization of methods for reducing greenhouse gases.
 Technical Optimization
Technical Optimization
The technical optimization of carbon dioxide (CO2) absorption after combustion using piperazine is an effective method for reducing greenhouse gases and improving air quality. Piperazine is recognized as a potent solvent in the CO2 absorption process, utilized in liquid absorption systems. Below are several key points in this regard:
Absorption Mechanism
As an organic solvent, piperazine reacts with CO2 to form stable complexes. This characteristic enhances the efficiency of CO2 absorption across various temperatures and pressures.
Optimal Conditions
To optimize the process, the conditions of temperature, pressure, and piperazine concentration must be precisely adjusted. Typically, lower temperatures and higher pressures result in increased absorption rates.
Renewability and Recovery
One of the main challenges in using solvents is their recovery after CO2 absorption. Optimizing the recovery process of piperazine can reduce costs and enhance the overall efficiency of the absorption system.
Research and Development
Further research in modifying the chemical structure of piperazine and combining it with other materials can aid in improving absorption efficiency and reducing costs.
In summary, the technical optimization of CO2 absorption using piperazine can be viewed as a sustainable and efficient solution for reducing greenhouse gas emissions and addressing climate change. The goal of optimization is to determine the optimal operational conditions to minimize energy consumption and maximize the volumetric efficiency of the absorption process. This optimization has been carried out for a range of flue gas (FG) compositions and varying CO2 absorption yields.
Economic Evaluation
Technical performance indicators serve as alternative cost indicators. Productivity and specific work equivalent are suitable indicators for investment costs and operational costs of solvent-based absorption processes. These indicators can effectively reflect the trends in investment costs and operational expenses.
Cost of Carbon Dioxide Capture
The minimum cost of capturing carbon dioxide per ton of CO2 captured is presented as a function of the flue gas flow rate for each combination of inlet gas concentration and CO2 absorption efficiency in the absorption process. An increase in the flow rate of the flue gas process leads to a reduction in the cost of capturing carbon dioxide due to economies of scale. However, this cost reduction continues only up to a certain threshold flue gas flow rate; beyond this point, further increases in flow rate can lead to increased costs.
An increase in CO2 absorption efficiency, especially at lower concentrations of CO2 in the inlet gas, results in a significant increase in the cost of capturing carbon dioxide.
Analysis of Various Cost Scenarios
The impact of changes in cost parameters, such as steam prices, electricity prices, and capital recovery rates on the cost of capturing carbon dioxide has been examined. Increasing steam prices up to three times the baseline value can raise the cost of capturing carbon dioxide by more than 60%. Similarly, increasing electricity prices up to three times the baseline value can increase the cost of capturing carbon dioxide by approximately 30%. Additionally, reducing the capital recovery rate to one-third of the baseline value can decrease the cost of capturing carbon dioxide by about 50%.
Conclusion
In this study, the technical and economic feasibility of post-combustion carbon dioxide absorption processes using aqueous piperazine has been examined.
The first noteworthy point is that an increase in carbon prices above 100 euros per ton of carbon dioxide makes the use of this absorption process economically viable for industrial sources with high carbon dioxide concentrations and large flue gas flows. Specifically, as carbon prices rise to 200 euros, optimal absorption efficiency increases to 84%, leading to a 47% increase in economic savings.
Even at lower carbon prices, such as 85 euros per ton, the use of this process for flue gas emission sources exceeding 1000 tons per day with high carbon dioxide concentrations will also be economically feasible, provided that excess heat recovery from these sources is implemented. This indicates that recovering and reusing excess heat can play a significant role in improving the economics of these processes.
Furthermore, the results show that under optimized steam and electricity costs, the cost of capturing each ton of carbon dioxide varies between 31 to 46 euros, depending on the concentration of carbon dioxide in the flue gas. An increase in carbon dioxide concentration up to 95% also leads to an increase in optimal absorption efficiency.
Examples of Completed Projects
Carbon Dioxide Simulation of Capture Using Ionic Liquids with Aspen Plus
Carbon Dioxide Simulation of Capture Using Enhanced Amines with Aspen Plus
Modeling CO2 Absorption from Air using Novel Solvents with Aspen Plus
Resources
Simulation of CO2 Capture After Combustion With Piperazine
In this project, the simulation, optimization, and economic assessment of carbon dioxide capture after combustion with piperazine in various flue gas compositions have been conducted using
Aspen Plus software.

 Technical Optimization
Technical Optimization