Introduction
In today’s industrial landscape, specialized chemicals such as para-xylene play a pivotal role. Para-xylene is a key component in producing polyesters and polyethylene terephthalates (PET), with growing global demand highlighting the need for efficient separation and purification processes. However, separating para-xylene from ethylbenzene in naphtha reformate is a significant chemical engineering challenge due to their close boiling points.
Traditional methods like solvent extraction and crystallization, although previously used, have limitations in efficiency and cost. One advanced method, the Parex process, utilizes adsorption technology and has achieved significant efficiency. However, its reliance on costly adsorbents and complex processes makes it suboptimal in all scenarios.
Project objectives
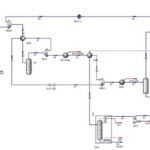
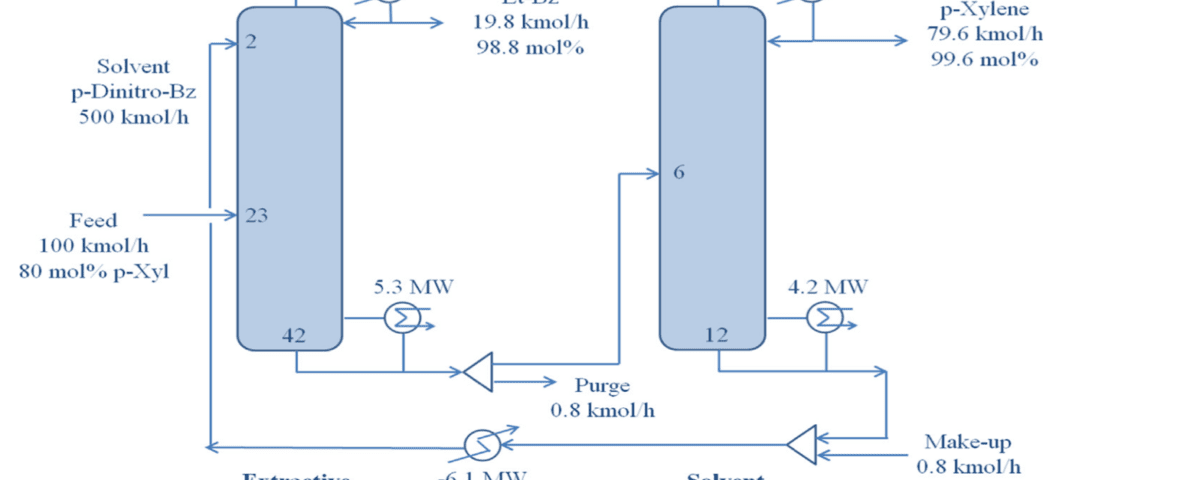
In this project, extractive distillation using p-dinitrobenzene as the extractant was introduced as a more efficient alternative. This solvent’s unique chemical properties and structural similarity to para-xylene enhance separation efficiency. The process design was modeled with HYSYS software and the NRTL thermodynamic model, and vapor-liquid equilibrium (VLE) data were validated using the advanced UNIFAC technique and molecular simulations.
The main objective of this study was to achieve a para-xylene purity of 99.6% with a recovery rate of 99.1%. economic analysis demonstrated that this innovative process is more cost-effective in terms of operational and investment expenses compared to conventional methods like the Parex process. This project showcases the potential of extractive distillation with p-dinitrobenzene to revolutionize separation processes in industrial applications.
Methodology and Tools
Solvent Selection and Properties
Selecting an appropriate extractant was critical. P-dinitrobenzene was chosen for its structural similarity to para-xylene and higher boiling point, which improves separation and simplifies operational complexity. This solvent enhances interactions with para-xylene, facilitating efficient separation from ethylbenzene.
Process Simulation
HYSYS software was utilized for process modeling and simulation. Using the NRTL model, the software provided precise analysis of vapor-liquid equilibrium (VLE) data, considering non-ideal solution behavior. Input parameters included feed composition, temperature, pressure, and solvent characteristics.
Vapor-Liquid Equilibrium (VLE) Validation
The UNIFAC technique was employed to calculate thermodynamic parameters for predicting phase behavior. Results from UNIFAC were compared against molecular simulations to ensure data accuracy and reliability.
Molecular Simulations
RASPA software was used for molecular simulations, enabling detailed modeling of molecular interactions. Simulations minimized energy configurations to establish thermodynamic equilibrium. Results validated the accuracy of the thermodynamic models and process design.
Process Optimization
Key parameters, including solvent flow rate, the number of distillation trays, and operational conditions, were optimized to maximize efficiency and reduce costs. Differential Evolution, an advanced numerical optimization algorithm, was used to determine optimal process conditions.
Tools Utilized
- HYSYS Software: For thermodynamic modeling and process simulation.
- RASPA Software: For molecular interaction simulations.
- UNIFAC Technique: To estimate VLE data.
- Optimization Algorithms: To refine operational parameters.
Key Results
Purity and Recovery
The process achieved a para-xylene purity of 99.6% and a recovery rate of 99.1%. These results underscore the effectiveness of p-dinitrobenzene as a solvent in extractive distillation, significantly improving selectivity and reducing complexity.
Economic Analysis
Economic comparisons showed that the proposed process is more competitive than traditional methods like the Parex process. By reducing operational costs, including energy consumption and eliminating expensive adsorbents, this method proves to be a cost-effective solution.
Role of Molecular Simulations
Molecular simulations played a vital role in optimizing the process. The structural similarity of p-dinitrobenzene to para-xylene enhanced intermolecular interactions, improving the separation factor. These simulations validated the solvent’s performance and supported the thermodynamic model.
Industrial and Operational Benefits
- Cost Reduction: Lower energy consumption and elimination of costly adsorbents.
- Higher Efficiency: Achieving high purity and recovery rates in industrial-scale applications.
- Environmental Compatibility: Minimizing chemical waste production.
This innovative process offers a sustainable and cost-effective alternative to traditional separation methods, making it highly viable for petrochemical industries.
Innovative Extractive Distillation for Para-Xylene Separation with HYSYS
SANILCO has successfully developed an innovative extractive distillation process for para-xylene separation, achieving 99.6% purity and 99.1% recovery. This method outperforms conventional techniques like the Parex process by reducing costs and operational complexity. Combining advanced modeling tools such as HYSYS, UNIFAC, and molecular simulations, this project demonstrates both scientific and economic advancements in industrial separation processes.